The Advanced Research Funding Gap: Three Concepts Too Breakthrough for Traditional Models
- Mijail Serruya
- Sep 30, 2025
- 10 min read
Why the most transformative BCI innovations fall through the cracks—and what it means for the future of neurotechnology
There's a peculiar paradox in neurotechnology funding: the projects with the highest potential for paradigm shifts are systematically unfundable through conventional channels. Not because they lack merit, scientific rigor, or commercial promise—but because they exist in a developmental twilight zone that traditional funding models weren't designed to support.
I call this the Advanced Research Funding Gap, and it's quietly holding back some of the most important work in brain-computer interfaces to launch forward and transform human wellbeing.
The Funding Valley of Death
Traditional venture capital seeks relatively de-risked technologies with clear 5-7 year exit pathways. Academic grants fund hypothesis-driven basic research with publications as primary deliverables. But what happens to breakthrough concepts that are:
Too applied for NIH/NSF basic research mandates (they fund hypothesis testing, not engineering development)
Too early for VCs seeking near-term returns
Too unconventional for institutional review committees
Too capital-intensive for angel investors
Too long-timeline for typical startup funding cycles
They die. Not from lack of scientific validity, but from structural incompatibility with existing funding architectures.
Three Case Studies in the Funding Gap
Let me make this concrete with three projects from my own research portfolio—each representing a different flavor of this systemic problem.
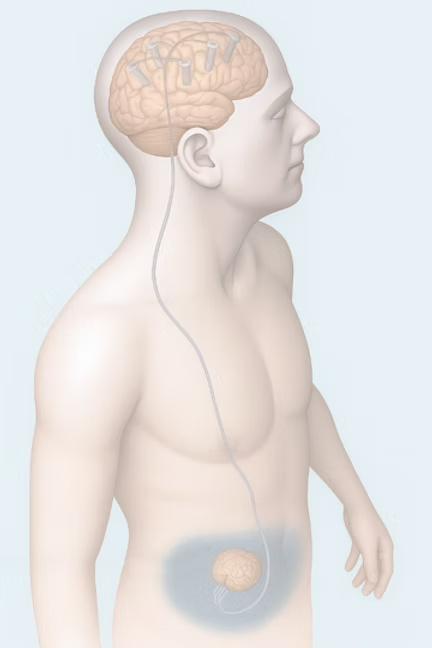
Project 1: The Fully Implantable Skull-Screw BCI (TRL 6-7)
The Innovation: Recording neural signals from metal screws anchored in the skull has been done for nearly a century with excellent signal quality. The technology fell out of favor for one simple reason: percutaneous devices that pierce the skin create infection risk. The breakthrough isn't the recording method—it's making it fully implantable, eliminating skin penetration while preserving the signal quality advantage of bone-conducted recordings.

Why Those Who Care About Scaling Neurotech Should Care: This perfectly addresses ARIA's specific focus on "massively scalable neurotechnologies" and their recognition that current approaches represent "a systems-level failure of profound consequence." Their program thesis explicitly seeks alternatives to craniotomies and dural penetration. While I wouldn't call making a scalp incision and drilling into skull bone completely "surgery-free," it can be done safely with conscious sedation rather than general anesthesia—scalable to procedure rooms the way endovascular procedures transformed cardiology - and with less risk of bleeding or blood clots than endovascular procedures. PCPs used to place Norplant in the office. Cardiologists routinely implant loop recorders in the chest. This belongs in that category: a procedure, not surgery.
Why It's Unfundable Traditionally:
Too engineering-focused for NIH/NSF: We're not testing hypotheses about neural mechanisms. We're solving a device engineering problem. Academic funders explicitly reject this kind of work.
Too medical for tech VCs: Requires regulatory pathway expertise and clinical development infrastructure most tech investors don't understand.
Too unconventional for medical VCs: "If this were possible, wouldn't Medtronic already be doing it?" (Classic innovator's dilemma thinking.)
Timeline mismatch: 2-3 years to proof-of-concept is too long for seed investors, too short to justify traditional biotech Series A dilution.
What It Needs: $2-5M for device miniaturization, hermetic sealing, biocompatibility validation, and initial human safety studies. A funding partner who understands that "massively scalable neurotech" requires rethinking surgical requirements, not just improving existing approaches.
Project 2: Living Neural Networks—A Three-Part Vision (TRL 4-5)
The Innovation: This is actually three breakthroughs in one, unified by a gaming platform that makes the entire vision economically viable:
Part 2A: The Gaming Platform as Research Engine A large-scale gaming platform where players interact with distributed biological neural tissue creates a crowdsourcing engine for mapping optimal input/output protocols. This simultaneously solves three problems:
For iBCI companies: Explores a massive state space of stimulation and decoding strategies that would be infeasible to test in living humans or even animal experiments. How do you best input data into neural tissue? How do you decode output? How do you leverage inherent neural plasticity through reinforcement learning?
For biocomputing: Maps how to communicate with neural tissue if you want to use it as computational substrate—not just recording what it does naturally, but training it to perform specific computations.
Revenue generation: The gaming platform creates immediate commercial value while solving fundamental research problems. Players pay to adorn their virtual pets; we learn how brains compute.
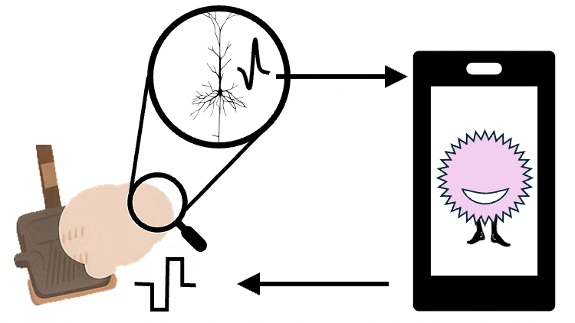
Part 2B: Living Amplifiers and Living Electrodes The fundamental limitation of all iBCI is that they're narrow sensorimotor pipes—they create artificial connectivity but can't recapitulate true computation. Instead of just reading from and writing to existing brain tissue, we purposefully modify the brain to make it easier to interface with.
My work with Kacy Cullen on living amplifiers and living electrodes recognizes what surgeons already do routinely: targeted nerve reinnervation, regenerative peripheral nerve interfaces, nerve and muscle transfers and grafts. We borrow skin, nerve, muscle, and blood vessels from one part of the body to repair another. We replace metal heart valves with porcine scaffolds. The question isn't whether we can do this—it's how to bring biological tissue engineering into iBCI.
Innervace raised $40M to make a living DBS but may have struggled with business model, just as Cyberkinetics was 20 years too early. The concept remains valid: living electrodes that grow with and adapt to the brain, reducing foreign body response and improving long-term stability. We can cap living electrodes with living amplifiers, and we can bioprint constructs to including multiplex and demultiplexing functionality.
Living constructs also have the advantage that they can be virally transfected (eg to become responsive to light or able to convey activity via ultrasound-readable signals) in vitro before being implanted, circumventing the risk of injecting viruses directly into the brain.

Part 2C: Neural Tissue as Brain Real Estate Expansion The distributed neural tissue in bioservers becomes literal real estate expansion of a person's brain. An iBCI connects not just to external computers but to living neural tissue in vitro that matches the computational properties of neural tissue in vivo. This is cognitive substrate expansion via cloud-connected bioservers—your brain scaling beyond your skull through biological computation, not just silicon.
Why It's Unfundable Traditionally:
Too weird for institutional science: Review committees struggle with "gaming platform as research vehicle" even though projects like FoldIt showed that crowdsourced citizen science can work and the fact that it's the fastest path to solving real-time bidirectional communication challenges.
Too biological for AI/ML investors: VCs funding neural networks mean artificial ones. They've watched Loihi, TrueNorth, FemtoSense, and Numenta fail to break into the transformer stack, and assume biological computing faces the same fate. They want to know a quick-return-problem for biocomputing that is analogous to what cryptography returns from quantum.
The energy efficiency trap: Yes, biological neural tissue is energy-efficient, but that alone won't displace silicon. You need either: (a) a specific use case where living biotissue outperforms neuromorphic silicon AND fits existing stacks (like Extropic or Great Sky are attempting), or (b) an entirely new, parallel biocompute stack that doesn't try to plug into von Neumann/cloud/transformer architectures modularity. Don't make a living GPU—make a new biocompute paradigm.
Vision scope: This requires an organization like e184 to either identify the narrow niche where biocompute wins now, or fund the long-term vision of an entirely parallel computational architecture.
What It Needs: $5-10M for platform development, biological culture optimization, closed-loop protocols, and initial commercial deployment. A funding partner who sees the three-part vision as integrated, not competing priorities.
Project 3: Cognitive Substrate Expansion—In-Body and Beyond (TRL 2-3)
The Innovation: This combines two breakthrough concepts into one unified vision:
In-Body Expansion: Peritoneal neural organoid clusters as external brain capacity—moving beyond "reading" or "writing" to actual cognitive expansion through distributed neural substrate within the body. Unlimited scaling beyond skull constraints while remaining within the body's immune envelope.
Beyond-Body Expansion: The Project 2 bioserver platform allows a person's brain to scale beyond their body entirely—cloud-connected biological neural tissue that serves as true computational extension, not just data storage or silicon processing.
A surgeon (human or robotic) can only fit so many implants and constructs within the skull. Yet if can refine implantable BCI- both synthetic and biological- then rather than just being a narrow pipe to recapitulate a simple sensorimotor loop, why not purposefully multiplex and link living-neural to living-neural to recapitulate living neural computation itself?
Further, it opens doors to create purpose-built interfaces to map abstract data into brain structures already evolved for multi-modality integration, such that we could one day perceive abstract data forms not as false-color or auditory interpolations, but in their own unique channels.


Together, these create a complete architecture: your brain scales within your body through ectopic neural tissue, and beyond your body through bioserver-hosted neural networks. Both use living neural tissue, both integrate via advanced iBCI, both transcend the physical limitations of the skull.
Why It's Unfundable Traditionally:
Too speculative for any VC: 5-10 year timeline to human applications. No venture fund has patience for that horizon on unproven biology.
Too applied for basic research: We're not asking "can organoids form neural networks?" (answered). We're asking "can they extend human cognition?" (engineering problem). NIH/NSF explicitly don't fund this.
Ethical complexity: Even though this is potentially transformative for cognitive restoration after injury, the "enhancement" implications make institutional funders extremely nervous.
Interdisciplinary demands: Requires expertise in neuroscience, tissue engineering, immunology, computational neuroscience, surgical technique, and distributed systems architecture—hard to review, harder to fund.
What It Needs: $10-20M over 5 years for organoid optimization, immune compatibility research, surgical technique development, bioserver integration protocols, and animal model validation. A funding partner with genuinely long-term vision who sees in-body and beyond-body expansion as complementary approaches to the same goal.
Why This Gap Matters
The advanced research funding gap isn't just concerning for researchers—it's systematically biasing neurotechnology development toward incremental improvements of existing paradigms.
Consider what gets funded easily:
Better electrode materials (incremental)
Improved signal processing algorithms (incremental)
Refined surgical techniques for established procedures (incremental)
Versus what struggles for funding:
Entirely new interface modalities
Biological computation paradigms
Cognitive architecture modifications
Living electrodes and amplifiers that modify the brain itself
The breakthrough innovations that could actually transform the field are structurally disadvantaged compared to safer, incremental work.
What Advanced Research Organizations Change
Organizations like e184 exist precisely to bridge this gap. They're designed for:
Long-term capital deployment: 3-10 year horizons matching actual innovation timelines
Technical risk tolerance: Understanding that breakthrough = uncertain, and that's okay
Interdisciplinary fluency: Evaluating projects that don't fit neat categorical boundaries
Mission alignment over exit pressure: Success = advancing the field, not just financial returns
Patient capital with strategic intent: Willing to fund the "valley of death" between proof-of-concept and commercial viability
Vision for paradigm shifts: Recognizing when a technology requires entirely new frameworks (like biocompute stacks) rather than incremental integration into existing systems
The Economic Case
Here's what makes this particularly frustrating: these projects have enormous commercial potential—just not on timeframes traditional investors understand.
Skull-screw BCIs could democratize neural interfaces by reducing cost and surgical complexity by orders of magnitude. Market: everyone currently using EEG because they can't access implantables.
Living neural networks could create entirely new computing paradigms with advantages in pattern recognition, adaptive learning, and human-computer symbiosis. Market: computational biology, drug discovery, adaptive gaming, therapeutic applications, and eventually a parallel biocompute industry.
Cognitive substrate expansion could fundamentally change human-AI collaboration and cognitive restoration after injury. Market: literally anyone with brain injury or degenerative disease or a brain that ages, that plus enhancement applications once the therapeutic pathway is established.
The total addressable market across these three concepts is tens of billions of dollars. But you can't fund them with traditional venture math because the timelines don't match investor return expectations, and the paradigm shifts require patient capital that believes in transformative vision.
What Breakthrough Innovation Actually Requires
After years navigating traditional funding models, I've learned what breakthrough neurotechnology development actually needs:
Capital Structure:
Patient, mission-aligned funding
5-10 year deployment timelines
Milestone-based tranches that respect actual research pace
Tolerance for pivots when biology surprises you
Organizational Support:
Interdisciplinary review committees who understand novel paradigms
Regulatory/IP strategy expertise for unprecedented technologies
Network connections to specialized manufacturing and clinical partners
Incorporating the voice of the people who can most benefit from the get go and intentionally welcoming and cultivating their critical thinking and creativity
Insulation from quarterly return pressure
Cultural Alignment:
Genuine excitement about technical risk
Understanding that "too early to tell if it will work" is not a disqualification
Recognition that breakthrough = uncertain, and uncertain ≠ invalid
Long-term thinking about field advancement, not just individual project ROI
Willingness to fund paradigm shifts that may require entirely new frameworks (like biocompute stacks) rather than incremental improvements
The Path Forward
The advanced research funding gap isn't insurmountable—it just requires purpose-built organizations that recognize breakthrough innovation as a distinct category requiring distinct support structures.
My three projects represent concrete examples of this systemic challenge. They're not edge cases—they're canaries in the coal mine, showing how current funding architectures systematically disadvantage the most transformative work.
The skull-screw BCI addresses ARIA's explicit call for massively scalable neurotech. Living neural networks solve the iBCI bandwidth problem while creating new biocompute paradigms. Cognitive substrate expansion—both in-body and beyond-body—transcends the fundamental physical limitations that constrain all current approaches.
If we want brain-computer interfaces to actually transform human capability rather than just incrementally improve existing paradigms, we need funding models that support genuine breakthroughs during their most vulnerable developmental stages.
That's what advanced research organizations do. That's what e184 does. And that's why the work of bridging this funding gap might be as important as any individual technical breakthrough.
The most important innovations in neurotechnology aren't being blocked by scientific challenges—they're being held back by financial architecture.
Imagine how we could help bring healing to previously inccurable conditions. Imagine what entirely novel computing architectures could emerge from the principled combination of brain-computer interfaces, in vitro neural constructs, artificial intelligence and massive, human crowdsourced wisdom, curiosity and creativity. Imagine how we could heal the substrate of our consciousness and pure awareness. Let's set this destination and set out to sea together.




Comments